Assessing Ground and Surface Water Quality in the Shenandoah Valley, Virginia
Overview: The goal of this Advanced Research Project is to equip upper level students with a deeper understanding of environmental geochemistry, fluvial sedimentology, and hydrology while contributing meaningful research on water contamination sources in the Shenandoah Valley, Virginia. Students will work on a variety of topics: aqueous geochemistry, sediment geochemistry, soil geochemistry, grain size analysis, and analyzing soil and sediment elemental and mineralogic compositions using scanning electron microscopy / energy dispersive X-ray spectroscopy (SEM/EDS), X-ray diffraction (XRD), X-ray absorption spectroscopy (XAS), and stable isotope mass spectrometry. Students will collaborate through field work, sample collection, data collection, and analysis to better understand what their research can tell us about water pollution. Together, the students will determine the distribution of trace metal contaminants in a variety of soils and sediments and their effects on nearby waters.
When: June 16 – July 14, 2021
Where: Washington & Lee University, Lexington, VA
Who: Eight rising seniors and project leaders Dr. Margaret Anne G. Hinkle (hinklem@wlu.edu) and Dr. Eva Lyon (elyon@wlu.edu), Washington & Lee University
Prerequisites and Recommended Courses: Preferably, all students will have completed Mineralogy and at least 1-2 semesters of chemistry or geochemistry. Courses in environmental science, geomorphology, sedimentology, or hydrology are a plus. ArcGIS or QGIS experience is required to participate in research topic #4 (see below).
Expectations and Obligations: Field sites are primarily reachable as day trips, so extensive camping experience is not needed. Sample collection will include digging pits, using soil corers, wading in streams, hauling field equipment, etc. Students should expect to contribute to this work and come prepared with field gear (boots, pack, hat, raingear, long pants, etc.).
Depending on the nature of what is ongoing with the COVID-19 pandemic, students may be required to be vaccinated 1 or more weeks prior to arrival, wear face masks when working with others inside or outside, and asked to quarantine for 2 weeks prior to the start of field work.
PROJECT DESCRIPTION
Introduction & Motivation
Iron (Fe) and manganese (Mn) (bio)geochemical cycling involves the oxidative precipitation of solid Fe(III) and Mn(III/IV) oxides and their reductive dissolution, producing aqueous Fe(II) and Mn(II). Fe(III) and Mn(III/IV) oxide minerals commonly occur in many environmentally and geologically relevant systems and typically exhibit large sorption capacities towards heavy metals (Cornell and Schwertmann, 2003; Liu et al., 2004; Post, 1999). Their prevalence, combined with their high sorption capacities, results in these minerals exerting substantial controls on the concentrations of heavy metals (often micronutrients at low concentrations but toxins at high concentrations) in waters, soils, and sediments (Brown et al., 1999; Brown and Parks, 2001; Fuller and Harvey, 2000; Hlawatsch et al., 2002; Jenne, 1968; Kumar et al., 2014; Lion et al., 1982; Manceau et al., 2002a, 2002b; Nelson and Lion, 2003; Peacock and Sherman, 2007; Smith and Longmore, 1980; Tebo et al., 1997; Young and Harvey, 1992). For example, arsenic (As) concentrations in groundwater are strongly linked to their adsorption to/desorption from Fe (hydr)oxides, and such processes may be responsible for the widespread As contamination of shallow groundwater wells in India and Bangladesh (Borch et al., 2010; Dixit and Hering, 2003; Islam et al., 2004) and even As concentrations in groundwater wells in Virginia (VanDerwerker et al., 2018).
Not only does the reductive dissolution of Fe(III) and Mn(III/IV) (oxyhydr)oxides lead to the release of sorbed heavy metals to the aqueous phase, it also causes increased Fe(II) and Mn(II) concentrations in solution. In the past decade, an ever increasing number of studies have found that Mn(II) in drinking water is a human health concern with wide ranging negative effects on IQ, infant mortality, and cancer rates, to name a few (Bjørklund et al., 2017; Bouchard et al., 2007, 2011; Hafeman et al., 2007; Khan et al., 2012; Langley et al., 2015; Sanders et al., 2014; Spangler and Spangler, 2009; Spangler and Reid, 2010; Williams et al., 2012). A recent study investigating Mn contamination in groundwater wells across the United States determined ~8% of groundwater wells in Virginia (out of 872 analyzed) have Mn concentrations ≥300 ppb (McMahon et al., 2018a), the lifetime chronic exposure health advisory limit for Mn set by the Environmental Protection Agency (U.S. Environmental Protection Agency, 2004). It has been argued that this health advisory limit is set too high (Ljung and Vahter, 2007), with just ≥100 ppb Mn in drinking water leading to some health and developmental effects (Langley et al., 2015; Sanders et al., 2014). Approximately 21% of groundwater wells in Virginia analyzed by McMahon et al. (2018a) have Mn concentrations exceeding that threshold (Figure 1). A majority of the population in over 30 Virginia counties rely on groundwater for their water supply (Figure 2), thus Mn may negatively impact the health of tens of thousands of Virginians.
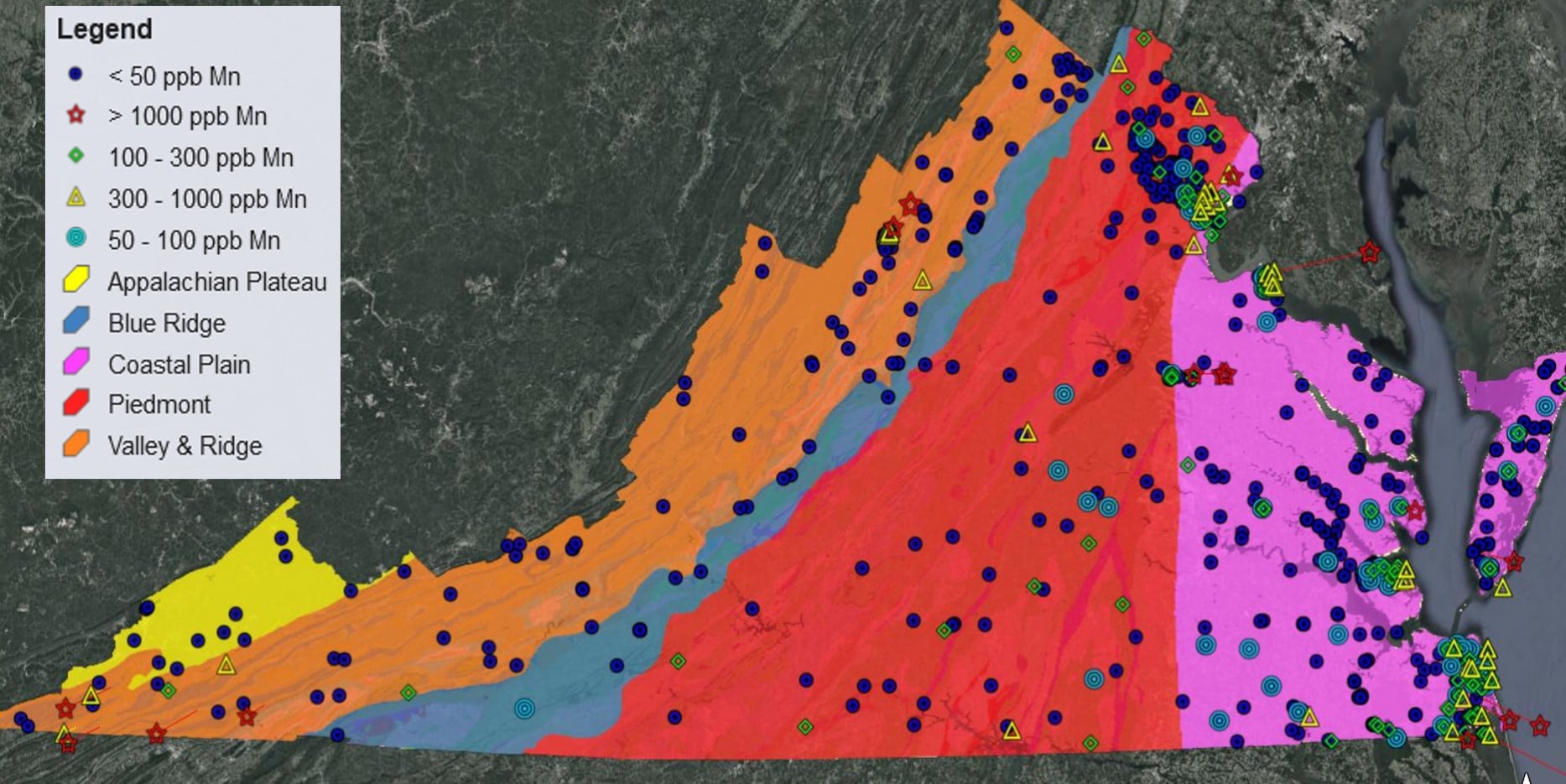
Figure 1: Mn well water data (obtained from McMahon et al., 2018a) plotted for the state of Virginia along with the Physiographic Provinces of Virginia, overlying the Virginia Geologic Map (Sources: McMahon et al., 2018a, Shufeldt et al., 2012).
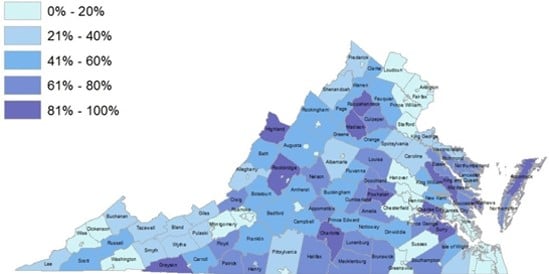
Figure 2: Percent of population in Virginia counties that rely on private wells for drinking water. (Source: Virginia State Water Resources Plan, 2015).
Mn contaminated groundwater wells have been identified in several locations throughout VA, including the upper portion of the Shenandoah Valley, VA (Fig. 1). Potential sources of Mn and trace metal contamination in VA groundwater include: dissolution of the aquifer, soil-water or sediment-water interactions, Mn mobilization via desorption from mineral surfaces in the soil-water interface, etc. Recent research on Mn contamination in the Roanoke river watershed points to mobilization of Mn associated with carbonate-rich rocks (Kiracofe et al., 2017). Two other recent studies investigating Mn groundwater contamination, one in the Piedmont region of North Carolina (Gillispie et al., 2016) and one surveying the entire United States (McMahon et al., 2018a) both found that soil geochemistry is strongly linked to Mn concentrations in groundwater. Thus, it is also possible that land use patterns may contribute to Mn contamination of groundwater. For example, disturbances from ore mining or the application of Mn-bearing fungicides or fertilizing biosolids could increase soil Mn concentrations and/or increase the dissolution of the initial soil Mn (Hue et al., 2001; Li et al., 2007; Semu and Singh, 1995), enhancing the mobilization of Mn and other associated trace metals from the solid to the aqueous phase. In order to predict the likelihood of Mn (or other trace metal) contamination in any drinking water source, identifying the major controls on their concentrations is critical.
We propose to investigate the role of land use patterns, soil geochemistry, aquifer type, and nearby stream compositions on Mn and other heavy metal concentrations in ground and surface waters in the Shenandoah Valley region of Virginia. This region was selected because of the high concentrations of Mn in several wells in this area (McMahon et al. 2018a), variable land use practices throughout the region, and potential for Mn and heavy metal inputs from historical ore mining operations. While there is already a substantial amount of well water data accessible via the USGS National Water Information Network (NWIN) and the Virginia Household Water Quality Program (VAHWQP), Mn concentrations in springs are not as well documented, even though springs are used as private drinking water sources. Thus, we propose to collect and analyze spring waters to add to the well water databases and to collect and analyze surface waters and soil cores near groundwater wells and springs.
Water compositions will be determined by a combination of ion chromatography (IC) and inductively-coupled plasma-mass spectrometry (ICP-MS) as well as in the field pH, dissolved oxygen, specific conductivity measurements, and alkalinity titrations. Soils will be analyzed by scanning electron microscopy paired with energy dispersive X-ray spectroscopy (SEM-EDS) and X-ray fluorescence to determine particle morphologies and general elemental compositions. Soil geochemistry will be further analyzed by total acid digestions and sequential extractions to determine the fractions that are exchangeable, adsorbed or associated with carbonates, adsorbed to Fe and Mn oxides, associated with organic matter (OM) and sulfide minerals, and the residual fraction. The speciation of Mn in the soils (i.e., does the Mn primarily occur as Mn oxides, bound to clays or Fe oxides, associated with OM, or in solution as aqueous Mn) will be determined by X-ray absorption near edge structure (XANES) spectroscopy at a synchrotron radiation light source. The role of geology and aquifer type will be assessed by plotting the wells and springs in ArcGIS to determine the underlying geology and correlate well depth with the specific aquifer.
Historical & Potential Future Impacts of Mill Pond Dams on Surface Water Quality
Increased aqueous Fe and Mn concentrations (and associated trace metals) in surface water is also linked with the stratification of impounded waters behind dams (Hess et al., 1989; Gordon, 1989; Dortch and Hamlin-Tillman, 1995; Ashby et al., 1999; Munger et al., 2017), negatively impacting surface water quality and surrounding ecosystems. In addition to these geochemical effects, another major consequence of dams includes reservoir infilling with sediments. These legacy sediments are stored behind dams that were constructed in the eastern United States from the earliest days of European settlement until the early 1900s (Walter and Merritts, 2008). Legacy sediments can potentially record evidence of land use changes to the land, such as urbanization and industrial and agricultural activities and thus can serve as important archives of colonial and antebellum land use history. The Shenandoah Valley is home to many historical low head dams (Fig. 3), primarily used for mills, iron forges, and other hydropower applications. Many of the dams have been breached over the years, either as a result of ongoing fluvial processes, aging infrastructure, or in response to calls for the removal of dams for a host of reasons, including their safety concerns (Fig. 4).
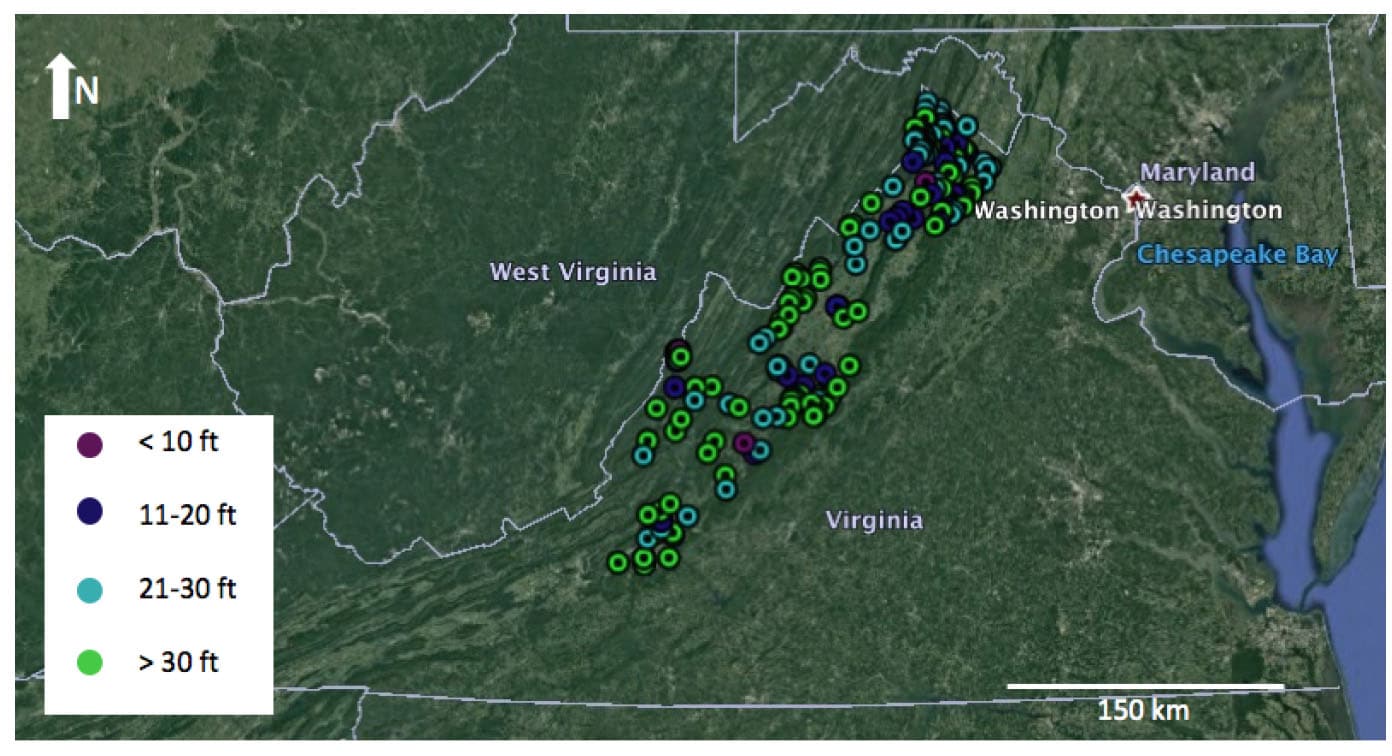
Figure 3: Dams registered with the National Inventory of Dams (NID) in the Shenandoah Valley cultural region. Most dams in the NID database for Virginia are greater than 30 feet in height, but these were mainly constructed after 1950. Lower head dams tend to be associated with the earlier period of mill dams (Source: Merritts et al., 2013). Figure constructed using Google Earth Satellite Imagery and NID data.
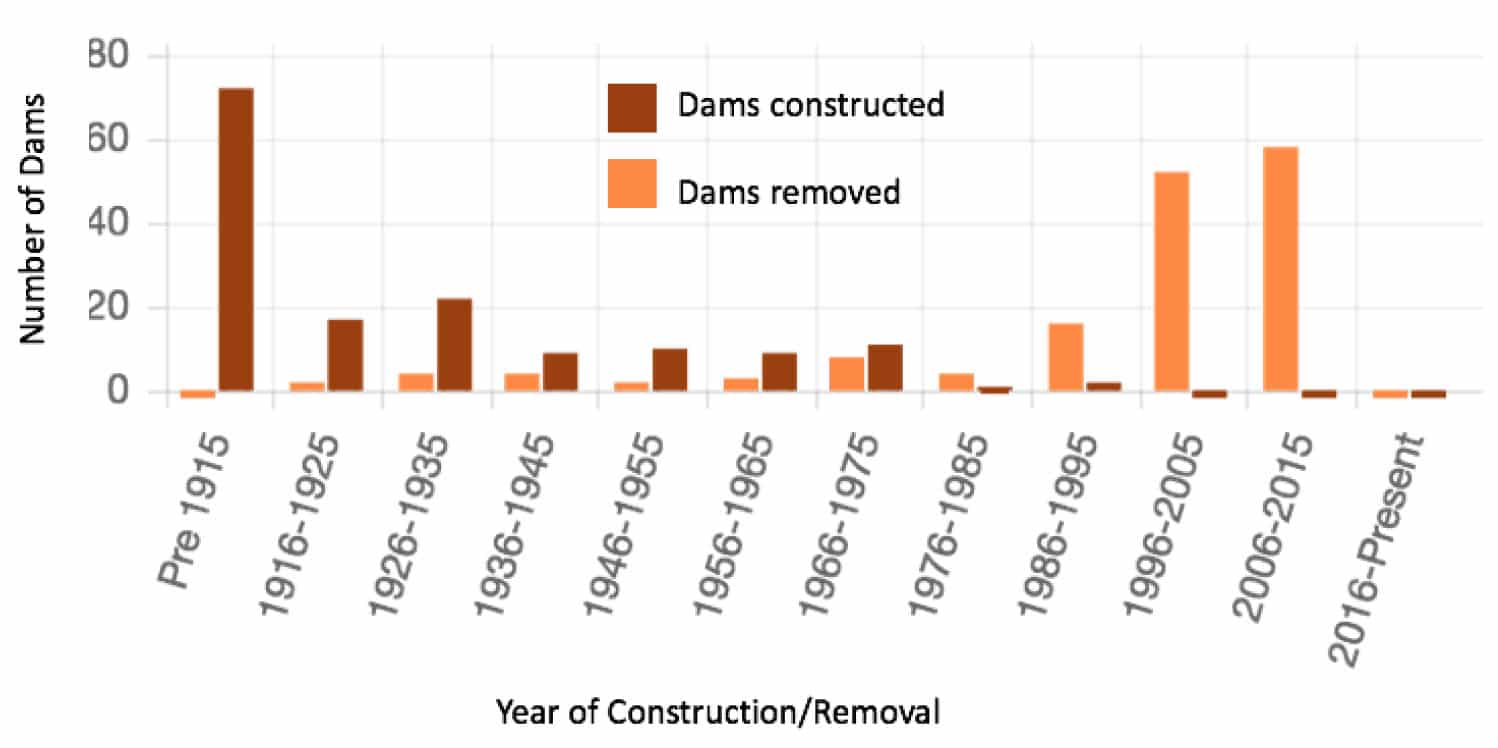
Figure 4: Trend of Dam Construction vs. Removal.(Source: USGS Dam Removal Information Portal).
When these dams are breached via dam failure, streams incise through these legacy sediments, re-mobilizing them and any potential contaminants (e.g., Walter and Merritts, 2008; Niemetz et al., 2012), negatively impacting downstream ecosystems via either geochemical contaminants or increased total suspended solids (TSS). Controlled dam removals, however, can mitigate these potential issues. We aim to characterize some of these legacy sediments at dams along the Maury River in Rockbridge County, Virginia. By characterizing the sediments at breached or removed dams in particular, we can better assess their potential for erosion into and contamination of the waterway, and downstream water bodies like the Chesapeake Bay.
The downstream transport of these legacy sediments from the Maury River has potential repercussions for the aquatic ecosystem of the Chesapeake Bay (e.g., Hupp et al., 2013; Niemetz et al., 2013). For example, sediment pollution negatively affects the benthic community in particular through several ways, including low dissolved oxygen, sediment contamination, and nutrient loading (Dauer et al., 2000). In addition to the issues of sediment pollution, these sediments can also contain adsorbed trace metals like Cu and Pb (Lutgen et al., 2020), especially when associated with fertilizers and pesticides (Cu) or emissions due to combustion of leaded gasoline (Niemetz et al., 2012). Lead in particular may be useful as an indicator of sediment age, given the peak in leaded gasoline use in the 1970s. By measuring such trace metals we can assess their potential contribution to the Maury River watershed.
Other geochemical indicators of interest include carbon and nitrogen, as their levels in pre-settlement sediments may be expected to have relatively high total organic carbon (TOC) and total nitrogen (TN), owing to the organic matter storage capacity of wetland soils (e.g., Walter and Merritts, 2008). We can therefore measure the bulk sediments for TOC and TN to identify the pre-settlement surface. Further, measuring sediment organic carbon and nitrogen isotope ratios may allow us to discern land use changes. For example, O’Leary (1981) found characteristic carbon isotope ratios for C3 and C4 plants, which differ in their photosynthetic pathways. Associated sediments may also record the isotopic shifts alongside the vegetation (Meyers and Ishiwatari, 1993; Meyers, 1994). Thus, it may be possible to distinguish the interval over which native vegetation (which is typically C3, unless grass or sedge) changes to crops like corn, maize, sorghum and others, which are C4 plants using the sedimentary record. Such a transition may be useful not only as a record of land use change in the watershed, but as a marker of sediment age. It should be noted that some crops like wheat, which was commonly grown in the Valley and Ridge Province in the 17th and 18th centuries, is a C3 plant (Hunter, 2005; Rood, 2014). Thus, the lack of a transition to carbon isotope ratios in the C4 plant range does not necessarily preclude a shift toward agriculture, while an identifiable shift from C3 to C4 confirms that such a shift occurred.
With this research on mill pond dams in the Shenandoah Valley, we propose to investigate the sediment and surface water trace metals, organic carbon content, and stable carbon isotopes, so that we may establish pre-settlement channel characteristics, as well as the history of fluvial sedimentation in the Maury River. Further, we can establish an erosion rate for stretches of the Maury River where channel geometry has previously been measured. Taken together, sediment characteristics and erosion rate will help us understand the potential for sediment pollution from legacy sediments. Fluvial sediments will be characterized based on grain size and composition using sieves, gravelometers, and either laser grain size analysis or hydrometer. These sediments will also be analyzed for stable isotope geochemistry (organic carbon and nitrogen) using elemental analysis and isotope ratio mass spectrometry (EA-IRMS) and trace metal chemistry using X-ray fluorescence (XRF) and SEM/EDS. Surface water geochemical compositions will also be assessed, with pH, DO, temperature, TSS, and specific conductivity measured in the field and samples collected for trace metal analyses with ICP-MS or -OES and major ions with IC. Water samples from several different depths will be collected and analyzed to determine if reservoir stratification has occurred. Channel geometry and bed and bank composition will be determined at sites of dam removal to establish rates of channel erosion.
Student Research Topics
All students will participate in field work, soil and water sample collection during the first two to three weeks of the summer research period. We expect that the students will gravitate toward one facet of the project and that, along with the student and their advisor’s background, will help them choose a specific topic to investigate more deeply. That said, students tasked with the XANES and EA-IRMS portions of the project will be supervised by Hinkle/Lyon. All students will learn scanning electron microscopy and/or X-ray fluorescence to use as needed for their project. Students will use these techniques to better understand soil and sediment morphology and composition. While we envision the possibility of these six projects to address the research questions at hand, some projects can be combined and all projects will share data and interpretations.
1. What is the geochemical composition of drinking water (surface, spring, and well water) with elevated Mn? With low to no Mn?
One student will analyze the data the group obtained from field measurements of the water chemistry (e.g., pH, dissolved oxygen, specific conductivity, and alkalinity titrations) associated with each site visited. The student will also prepare collected water samples and standards for ICP-MS (for 27 elements: Mn, Fe, As, antimony, barium, beryllium, lead, mercury, thallium, nickel, chromium, phosphorous, sulfur, strontium, cadmium, cobalt, zinc, copper, fluoride, aluminum, titanium, zircon, boron, silver, lithium, silicon, and selenium) and IC analysis (for cations like sodium and magnesium and anions like phosphate, sulfate, and nitrate) as well as analyze and interpret the data they obtain. It is quite possible that there will be correlations between the aqueous Mn and other trace metals, as has been identified for Mn and As in well waters in North Carolina (Sanders et al., 2014). In the Coastal Plain region of Virginia, it has already been documented that there are positive correlations aqueous Mn and hard water (water with high concentrations of calcium, magnesium, and carbonate ions due to the dissolution of carbonate host rocks) in wells (McFarland, 2010), and it is possible that this trend may be consistent across the state. The data from these analyses will be paired with their geographic location and well depth so that water contamination can be spatially resolved across the state.
2. What is the soil composition near waters that have characteristically high Mn concentrations? Does that differ from soils near waters with low/non-existent Mn?
One student will perform sequential extractions and complete acid digestions on soil samples collected from sites near water sources with Mn concentrations > 100 ppb and Mn concentrations < 50 ppb. This student will also prepare standards and samples for analysis by ICP-OES and analyze the ICP-OES data. Their work will identify what contaminants are present in the soils (if any), their relative concentrations, and how labile those contaminants are. If, as it is suggested by Gillispie et al. (2016) and McMahon et al. (2018a), weathering from soils is correlated with Mn contamination in groundwater, this work will serve to help identify the extent of that process in Virginia drinking waters.
3. What is the speciation of Mn (and other important trace elements) in the soils?
One student will be in charge of preparing samples for XANES measurements and attending Beam Time at the synchrotron with Hinkle and other interested students in Fall 2021 / Winter 2022. This student will gain experience in synchrotron-based science at a national lab. Soils from sites near water sources with Mn concentrations > 100 ppb and Mn concentrations < 50 ppb will be analyzed with XANES to determine if Mn speciation is different in soils near waters with elevated aqueous Mn. For example, it is possible that soils near waters with low aqueous Mn concentrations may contain Mn as solid Mn oxides, while soils near waters with higher aqueous Mn concentrations may contain more adsorbed Mn. The student will also learn how to prepare standards for the XANES measurements (some of which includes mineral syntheses), the basics on normalizing and processing XANES spectra, and the fundamentals of fitting data.
4. Are there correlations between trace metal concentrations in drinking water and the geologic, pedologic, hydrologic, or land use history of that site?
One student will be responsible for maintaining the database for water and soil geochemical data, paired with the site’s geographic location. Using ArcGIS and well depth information, students will identify the surface geology, physiographic province, soil type, distance to the nearest surface water body, and aquifer for each site, and plot each site with its corresponding attribute table (with water and soil geochemical data). They will generate maps with our data on trace metal contamination (such as Mn, As, or lead) as well as data from other sources such as the USGS NWIN and the VAHWQP. If the student is particularly motivated and has strong support from an academic advisor interested in logistic regression modeling, they can use the data to determine if any relationships exist between geologic, pedologic, or hydrologic settings and trace metal concentrations in the groundwater, as has been done for As in Virginia (VanDerwerker et al., 2018).
5. What are the sedimentological characteristics of the legacy sediments?
One student will characterize legacy sediments in terms of their grain size and composition. This will be accomplished by measuring bank stratigraphy in the field using traditional methods, determining grain size for the coarse fraction using sieves and/or a gravelometer, and measuring the fine fraction with a Mastersizer or other laser-based analytical instrument. If such an instrument is unavailable, we will de-floc the clays and use a hydrometer to determine clay/silt size fraction.
6. What are the geochemical characteristics of the legacy sediments and surface waters?
One student will be tasked with assessing the geochemical characteristics of the legacy sediments and surface waters, in order to identify any potential future sources of water contamination and ongoing issues. This student will conduct total acid digestions of the legacy sediments and a few select sequential extractions to obtain information regarding the geochemical makeup of those sediments. The student will prepare ICP-OES standards and samples for analysis and analyze the resulting data. The sediments will be further characterized for mineralogy via XRD as well as particle morphology and general geochemistry via SEM/EDS and XRF. This student will also analyze the data the group obtains from the field mill pond water chemistry measurements (e.g., pH, dissolve oxygen, specific conductivity, and alkalinity titrations) at different depths, which will allow us to identify any stratification that may be occurring in the mill ponds. The student will also prepare water samples and standards for ICP-MS analyses (for 27 elements: Mn, Fe, As, antimony, barium, beryllium, lead, mercury, thallium, nickel, chromium, phosphorous, sulfur, strontium, cadmium, cobalt, zinc, copper, fluoride, aluminum, titanium, zircon, boron, silver, lithium, silicon, and selenium) and IC analysis (for cations like sodium and magnesium and anions like phosphate, sulfate, and nitrate) and interpret the data they obtain.
7. What do the legacy sediments reveal about the land use history of the region?
A student will investigate if the sediments impounded behind the mill pond dams retain isotopic geochemical information regarding the land use history of the site. Specifically, the student will measure the percent total organic carbon and nitrogen, and stable isotopes of C and N for bulk sediments using an elemental analyzer coupled to an IRMS (EA-IRMS). Total organic carbon can be measured on the EA after acid digestion of the sediments to remove inorganic carbon.The student will be responsible for both preparing standards and samples and using the resulting data to make interpretations regarding the suitability of mill pond sediments for assessing past shifts in plant cover.
8. What is the volume of legacy sediments entering the Maury River on an annual basis?
After characterizing the nature of the sediments, it is important to determine their potential for stream contamination, especially if the sediments contain significant levels of trace metals or other contaminants. Therefore, at least one student will work on establishing a bank erosion rate by measuring channel geometry and other stream properties, including flood recurrence, bankfull flow, bank strength, and critical stream power. Using measurements of channel geometry from immediately after, and two years after removal of the Jordan’s point dam, we will attempt to quantify the amount and rate of sediment erosion the legacy sediments impounded behind such structures along the Maury River. Iosso (2020) found little fine sediment storage behind the low head structure, but calculated the volume of legacy sediment depths from bank and floodplain sediments. Merritts et al. (2013) find that erosion rates decelerate with time after dam removal, so an erosion rate established after dam removal in 2019 would differ from that in 2021, and would likely differ from future rates. Calculating the present erosion rate is an essential first step in determining the future potential for sediment pollution from this and other such dams.
Learning Outcomes
This project will enable students to apply geoscience topics to real-world environmental problems. Students will become the group’s experts on the techniques involved for their specific topic selection and the data resulting from it. As we continue to collaborate over the academic year they will be exposed to the data and findings of the rest of the group and will better understand the strengths and weaknesses of each technique. They will also gain experience using research, literature review, and collaboration to build a cohesive scientific narrative. At the end of the project we expect students to:
- Gain proficiency in field sampling techniques for soil, sediments, and/or water – including maintaining good field notes.
- Practice proper sample storage and preparation techniques.
- Carry out a variety of measurements and properly manage the data they acquire.
- Use computational tools to analyze their data and collaborate with others to interpret these findings.
- Use a variety of techniques including posters, student theses, and journal articles to effectively communicate their findings – within the group and with others.
- Understand the gravity of ethics in research and responsible conduct in the field and the laboratory
- Learn about and practice the collaborative aspects of science
- Gain an appreciation for the interdisciplinary nature of geoscience, gained by combining environmental geochemistry with geophysics to understand contaminant dynamics in drinking waters.
Students will also gain a variety of technical skills, which may vary depending on the project selection:
- Use of Excel, MATLAB, and/or R for data processing and creating high quality graphs
- Reading maps and determining locations based on GPS
- ArcGIS skills in mapping field sites
- Sample preparation techniques for soil, sediment, and water sample analyses, and/or X-ray absorption spectroscopy
- Making standards and samples for EA-IRMS, IC, ICP-OES, and ICP-MS
- Sequential extraction of soils
- Field skills for sediment and water sampling, water analysis, and soil coring
- SEM/EDS and XRF analyses of soils
- Grain size analysis of sediments
- EA-IRMS analysis of sediments
PROJECT LOGISTICS
- Week 1: Students arrive at Washington & Lee University, readings and introduction to the project and region, selecting project, training in sample collection and begin sample collection at field sites.
- Week 2: Sample collection at field sites.
- Week 3: Complete sample collection at field sites, begin sample prep and lab analyses at W&L.
- Week 4: SEM/EDS, analyzing field data, preliminary analyses, abstract writing at W&L.
After the summer, during the academic 2021-2022 year, students will continue progressing with data collection and analysis as stipulated by their research proposals and timelines. We will touch base in a monthly whole-group videoconference and arrange to check in with individual students and their home advisor (when possible) every month (or as needed) so that goals are met and any issues are addressed.
Planning for the COVID-19 Era
If we have learned anything during 2020, it is that flexibility is key to everything. Hinkle’s motto throughout this pandemic has been “prepare for the worst, but hope for the best.” For this project, “preparing for the worst” means we need to entertain three potential scenarios:
- Fully in-person. The project runs as outlined above, taking the precautions necessary to minimize risk of coronavirus to all participants.
2. Hybrid. It may be possible that outside researchers may not be allowed on campus next summer, as happened this past summer. If that occurs, there are one of two possible options: 1. If we know about this restriction far ahead of time, Hinkle’s Environmental Field Methods course (an intensive lab course taught in the W&L Spring Term of 2021 – a 4 week term in which students only take that one course) can do a large portion of the field work; 2. If there is not much warning, then a W&L student (and Keck participant) will start about two weeks earlier than originally planned dates to conduct all the field work. Then, we can either send samples out to the other students and they can analyze them at their home institutions, or we can send the samples to other labs for analysis, and the Keck students can work with the data we receive back from those labs.
3. Fully Virtual. There is the chance we may need to prepare to be fully virtual. However, we expect the ‘hybrid’ option to be the most likely, as even last summer students were permitted to conduct field work with faculty members (but were not allowed inside labs/buildings to work) at W&L. That said, personal health or other issues may arise (2020 certainly has taught me that nothing is certain), in which case we do need to plan for a fully virtual summer. If this is the case, Hinkle has a backlog of samples on spring waters collected in Rockbridge County – the students can use the geochemistry data from these samples in a similar manner to what has already been described. Hinkle’s current thesis student has been working on creating a geospatially referenced database of Mn concentrations in groundwater across the Shenandoah Valley. Using what she has created, the Keck students can build on that database, and work on creating a similar database in other regions of interest, such as Southwest Virginia and Pennsylvania – or perhaps Mn in a region of interest to them.
PROFESSIONAL DEVELOPMENT
Students will present their findings at the Geological Society of America 2021 Annual Meeting. Students will work together in teams of at least two to write abstracts and design posters for these presentations. We will also take this time to discuss results, touch base on progress and the timeline for completing the Advanced projects. We will also discuss career plans and paths forward with the students to better support them in their career goals. Students will participate in student activities at these meetings and attend talks in their areas of interest.
All students will submit a short contribution to the Proceedings of the Keck Geology Consortium 2022 Volume, with drafts due to their home advisor by Mid-February 2022, revised drafts to the project directors by March 1st, 2022, with the final paper submitted in Mid-March 2022.
References
Appelo, C.A.J., Postma, D., 2004. Geochemistry, Groundwater and Pollution, 2nd ed. CRC Press LLC.
Ashby, S. L., Myers, J. L., Laney, E., Honnell, D., & Owens, C. (1999). The effects of hydropower releases from Lake Texoma on downstream water quality. Journal of Freshwater Ecology, 14, 103–112.
Carmichael, S.K., Doctor, D.H., Wilson, C.G., Feierstein, J., McAleer, R.J., 2017. New insight into the origin of manganese oxide ore deposits in the Appalachian Valley and Ridge of northeastern Tennessee and northern Virginia, USA. GSA Bulletin 129, 1158–1180.
Dauer, D. M., Ranasinghe, J. A., & Weisberg, S. B. 2000. Relationships between benthic community condition, water quality, sediment quality, nutrient loads, and land use patterns in Chesapeake Bay. Estuaries, 23, 80-96. https://doi.org/10.2307/1353227
Dortch, M. S., & Hamlin‐Tillman, D. E. (1995). Disappearance of reduced manganese in reservoir tailwaters. Journal of Environmental Engineering, 121, 287–297.
Fechter, L.D., 1999. Distribution of manganese in development. Neurotoxicology 29, 197–201.
Gillispie, E.C., Austin, R.E., Rivera, N.A., Bolich, R., Duckworth, O.W., Bradley, P., Amoozegar, A., Hesterberg, D., Polizzotto, M.L., 2016. Soil Weathering as an Engine for Manganese Contamination of Well Water. Environmental Science & Technology 50, 9963–9971. https://doi.org/10.1021/acs.est.6b01686
Gordon, J. A. (1989). Manganese oxidation related to the releases from reservoirs. Journal of the American Water Resources Association, 25, 187–192.
Guidelines for Drinking-water Quality, 2017. . World Health Organization, Geneva.
Hafeman, D., Factor-Litvak, P., Cheng, Z., van Geen, A., Ahsan, H., 2007. Association between Manganese Exposure through Drinking Water and Infant Mortality in Bangladesh. Environmental Health Perspectives 115, 1107–1112. https://doi.org/10.1289/ehp.10051
Hess, G. W., Kim, B. R., & Roberts, P. J. W. (1989). A manganese oxidation model for rivers. Journal of the American Water Resources Association, 25, 359–365. doi:10.1111/j.1752‐1688.1989.tb03072.x
Hupp, Cliff R., Noe, G. B., Schenk, E. R., & Benthem, A. J. 2013. Recent and historic sediment dynamics along Difficult Run, a suburban Virginia Piedmont stream. Geomorphology, 180–181, 156–169. https://doi.org/10.1016/j.geomorph.2012.10.00
Hlawatsch, S., Garbe-Schönberg, C.D., Lechtenberg, F., Manceau, A., Tamura, N., Kulik, D.A., Kersten, M., 2002. Trace metal fluxes to ferromanganese nodules from the western Baltic Sea as a record for long-term environmental changes. Chemical geology 182, 697–709.
Hunter, B. 2005., Wheat, War, and the American Economy during the Age of Revolution. The William and Mary Quarterly, 62, 505-526. https://doi.org/10.2307/3491533
Iosso, C. 2020. Impacts of Low-Head Dam Construction and Removal with Little Channel Sediment Storage: Case Study from the Maury River, Virginia. https://dspace.wlu.edu/xmlui/bitstream/handle/11021/34927/WLURG38_Iosso_GEOL_2020.pdf?sequence=1&isAllowed=y
Kalbian, M., & Pezzoni, J., 2019. Removal report on Jordan’s Point concrete dam and timber crib dam : May 23-25, and May 28-30, 2019. https://www.dhr.virginia.gov/wp-content/uploads/2020/12/RB-103_Removal_Report_Jordans_Point_Dams_2019_KALBIAN_report.pdf
Lutgen, A., Jiang, G., Sienkiewicz, N., Mattern, K., Kan, J., & Inamdar, S. 2020. Nutrients and Heavy Metals in Legacy Sediments: Concentrations, Comparisons with Upland Soils, and Implications for Water Quality. Journal of the American Water Resources Association. 56, 669-691.
Major, J. J., East, A. E., O’Connor, J. E., Grant, G. E., Wilcox, A. C., Magirl, C. S., Collins, M. J., Tullos, D. D. 2017. Geomorphic responses to dam removal in the United States – a two-decade perspective, in Gravel bed rivers: Processes and disasters. Wiley. 355-383.
Merritts, D., Walter, R., Rahnis, M., Hartranft, J., Cox, S., Scheid, C., Potter, N., Jenschke, M., Reed, A., Matuszewski, D. and Kratz, L. 2013. The rise and fall of Mid-Atlantic streams: Millpond sedimentation, milldam breaching, channel incision, and stream bank erosion. The challenges of dam removal and river restoration, 21st edn. Geological Society of America Reviews in Engineering Geology, Boulder, 183-203. https://doi.org/10.1130/2013.4121(14)
Meyers, P.A., 1994. Preservation of elemental and isotopic source identification of sedimentary organic matter. Chemical Geology, 114, 289-302.
Meyers, P. A., & Ishiwatari, R., 1993. Lacustrine organic geochemistry—an overview of indicators of organic matter sources and diagenesis in lake sediments. Organic geochemistry, 20, 867-900.
Montgomery, D.R., 2007. Soil erosion and agricultural sustainability. Proceedings of the National Academy of Sciences 104, 13268–13272. https://doi.org/10.1073/pnas.0611508104
Munger, Z. W., Shahady, T. D., Schreiber M.E. (2017) Effects of reservoir stratification and watershed hydrology on manganese and iron in a dam‐regulated river. Hydrological Processes, 31, 1622-1635.
National Inventory of Dams. Army Corps of Engineers. Accessed December 2020. https://nid.sec.usace.army.mil/ords/f?p=105:22:9336801692043::NO:::
National Primary Drinking Water Regulations, 2009.
Niemitz, J., Haynes, C., & Lasher, G., 2013. Legacy sediments and historic land use: Chemostratigraphic evidence for excess nutrient and heavy metal sources and remobilization. Geology, 41, 47-50. https://doi.org/10.1130/G33547.1
O’Leary, M. H., 1981. Carbon isotope fractionation in plants. Phytochemistry, 20, 553-567. https://doi.org/10.1016/0031-9422(81)85134-5
Rood, D., 2014. Bogs of Death: Slavery, the Brazilian Flour Trade, and the Mystery of the Vanishing Millpond in Antebellum Virginia. The Journal of American History, 101, 19-43. https://doi.org/10.1093/jahist/jau301
Trout, W., 1991. The Maury River atlas: Nineteenth-century inland navigations of the Virginias . Lexington, Va: The Canals and Navigation Society.
U.S. Environmental Protection Agency, 2004. Drinking Water Health Advisory for Manganese.
United States Geological Survey Dam Removal Information Portal (DRIP). United States Geological Survey Accessed December 2020. https://www.sciencebase.gov/drip/
Virginia State Water Resources Plan, 2015.
Walter, R. C., & Merritts, D. J. 2008. Natural streams and the legacy of water-powered mills. Science, 319, 299-304. https://science.sciencemag.org/content/319/5861/299.abstract?casa_token=RPEUI9lfJ2IAAAAA:mSku-57PH8m8ISWq3qiStQkhkwYXTHBt3iJVZJ6Oi6wWrlx4Ga9LA3o_lmkvnFOMJ5ejiydPfbkCBoo
Yochum, S. E., 2018. Guidance for stream restoration. US Department of Agriculture, Forest Service, National Stream & Aquatic Ecology Center. https://riversedgewest.org/sites/default/files/resource-center-documents/yochumusfs-nsaec-tn102-2gudncstrmrstrtnrhbltn.pdf
Young, L.B., Harvey, H.H., 1992. The relative importance of manganese and iron oxides and organic matter in the sorption of trace metals by surficial lake sediments. Geochimica et Cosmochimica Acta 56, 1175–1186. https://doi.org/10.1016/0016-7037(92)90055-N
Zaw, M., & Chiswell, B. (1999). Iron and manganese dynamics in lake water. Water Research, 33, 1900–1910.

