Identifying major controls on soil and groundwater contamination in the Shenandoah Valley & Southwest Virginia
Overview: This eight student Advanced Research Project will investigate the role of land use patterns, soil geochemistry, aquifer type, and nearby stream compositions on manganese (an important micronutrient at low concentrations but a contaminant with devastating health effects at high concentrations) and other heavy metals in ground and surface waters in the Shenandoah Valley and Southwest regions of Virginia. Recent research by the USGS has identified several wells in these two areas as having high manganese concentrations. Exposure to aqueous manganese via drinking water is associated with a wide range of neurodevelopmental and health effects, such as lowered IQ, increased infant mortality, and increased incidence of cancer. Manganese concentrations in drinking water are often dependent on redox gradients, with manganese most typically stable in the aqueous phase under reducing environments (such as groundwater) or in acidic conditions (such as coal mine drainage systems, with many located in Southwest Virginia). Manganese contamination in subsurface water sources can be due to soil weathering (which increases with certain agricultural practices), aquifer dissolution, desorption of Mn from mineral surfaces, or inputs from mining pollution. In order to predict the likelihood of manganese (or other heavy metal) contamination in any drinking water source, identifying the major controls on their concentrations is critical. Students will collect and analyze spring and surface water samples with a variety of geochemical techniques to add to the growing available groundwater well databases, collect and analyze soil cores near water sample sites, and identify past land use patterns with magnetic study. By combining aqueous geochemistry with soil geochemistry, sediment magnetism, imaging, synchrotron-based X-ray absorption spectroscopy, and GIS, we aim to identify the major sources of manganese contamination across the region.
When: June 21 – July 18, 2020 (tentative)
Where: Washington & Lee University, Lexington, VA
Who: Eight rising seniors and project leaders Dr. Margaret Anne G. Hinkle (hinklem@wlu.edu), Washington & Lee University, and Dr. Anna Lindquist (alindqu1@macalester.edu), Macalester College
Prerequisites and Recommended Courses: Preferably, all students will have completed Mineralogy and at least 1-2 semesters of chemistry or geochemistry. Environmental science courses and hydrology are a plus. Two semesters of physics is preferred, but not necessary, for the students involved in the magnetic studies. ArcGIS experience is required to participate in that research topic. Field sites are primarily reachable as day trips, so extensive camping experience is not needed. Sample collection will include digging pits, using soil corers, wading in streams, hauling field equipment, etc. Students should expect to contribute to this work and come prepared with field gear (boots, pack, hat, raingear, long pants, etc.). We are especially interested in applicants who are interested in environmental geochemistry or geophysics, who enjoy working in the field and in the laboratory, and are comfortable working both in groups and independently. It is highly recommended that students select an advisor with expertise in low-temperature geochemistry, soil science, hydrology, or geophysics. Students who plan to use this research for a senior thesis (or equivalent) will be given preference.
Expectations and Obligations:
1. Participation in all field work, data collection, and activities during the designated summer period (June 21 – July 18, 2020).
2. Follow up data analyses at home institution, sharing data via the designated data management plan, and regular correspondence and videoconferencing calls with the research team (with student partners as well as with project leaders) throughout the 2019-2020 academic year.
3. If in Team 1 or Team 2, short travel during late Summer 2020 or Fall 2020 to collect data at National Laboratories.
4. Write an abstract and present research findings (as either a poster or a talk) at either the Geological Society of America Annual National Meeting (in Montreal, Quebec, Canada in October 25- 28, 2020) or the American Geophysical Union Fall Meeting (in San Francisco, CA, USA in December 7 – 11, 2020).
5. Write an article for the Proceedings of the Keck Geology Consortium 2021 Volume (first draft due to home advisors Mid-February 2021 and revised version to project directors by March 1, with the final version submitted to the Keck Office in Mid-March, 2021).
6. Expected but not required: Use this research to complete a senior thesis (or equivalent) at your home institution.
PROJECT DESCRIPTION
Introduction & Motivation
Iron (Fe) and manganese (Mn) (bio)geochemical cycling involves the oxidative precipitation of solid Fe(III) and Mn(III/IV) oxides and their reductive dissolution, producing aqueous Fe(II) and Mn(II). Fe(III) and Mn(III/IV) oxide minerals commonly occur in many environmentally and geologically relevant systems and typically exhibit large sorption capacities towards heavy metals (Cornell and Schwertmann, 2003; Liu et al., 2004; Post, 1999). Their prevalence, combined with their high sorption capacities, results in these minerals exerting substantial controls on the concentrations of heavy metals (often micronutrients at low concentrations but toxins at high concentrations) in waters, soils, and sediments (Brown et al., 1999; Brown and Parks, 2001; Fuller and Harvey, 2000; Hlawatsch et al., 2002; Jenne, 1968; Kumar et al., 2014; Lion et al., 1982; Manceau et al., 2002a, 2002b; Nelson and Lion, 2003; Peacock and Sherman, 2007; Smith and Longmore, 1980; Tebo et al., 1997; Young and Harvey, 1992). For example, arsenic (As) concentrations in groundwater are strongly linked to their adsorption to/desorption from Fe (hydr)oxides, and such processes may be responsible for the widespread As contamination of shallow groundwater wells in India and Bangladesh (Borch et al., 2010; Dixit and Hering, 2003; Islam et al., 2004) and even As concentrations in groundwater wells in Virginia (VanDerwerker et al., 2018).
Not only does the reductive dissolution of Fe(III) and Mn(III/IV) (oxyhydr)oxides lead to the release of sorbed heavy metals to the aqueous phase, it also causes increased Fe(II) and Mn(II) concentrations in solution. In the past decade, an ever increasing number of studies have found that Mn(II) in drinking water is a human health concern with wide ranging negative effects on IQ, infant mortality, and cancer rates, to name a few (Bjørklund et al., 2017; Bouchard et al., 2007, 2011; Hafeman et al., 2007; Khan et al., 2012; Langley et al., 2015; Sanders et al., 2014; Spangler and Spangler, 2009; Spangler and Reid, 2010; Williams et al., 2012). A recent study investigating Mn contamination in groundwater wells across the United States determined ~8% of groundwater wells in Virginia (out of 872 analyzed) have Mn concentrations ≥300 ppb (McMahon et al., 2018a), the lifetime chronic exposure health advisory limit for Mn set by the Environmental Protection Agency (U.S. Environmental Protection Agency, 2004). It has been argued that this health advisory limit is set too high (Ljung and Vahter, 2007), with just ≥100 ppb Mn in drinking water leading to some health and developmental effects (Langley et al., 2015; Sanders et al., 2014). Approximately 21% of groundwater wells in Virginia analyzed by McMahon et al. (2018a) have Mn concentrations exceeding that threshold (Figure 1). A majority of the population in over 30 Virginia counties rely on groundwater for their water supply (Figure 2), thus Mn may negatively impact the health of tens of thousands of Virginians.
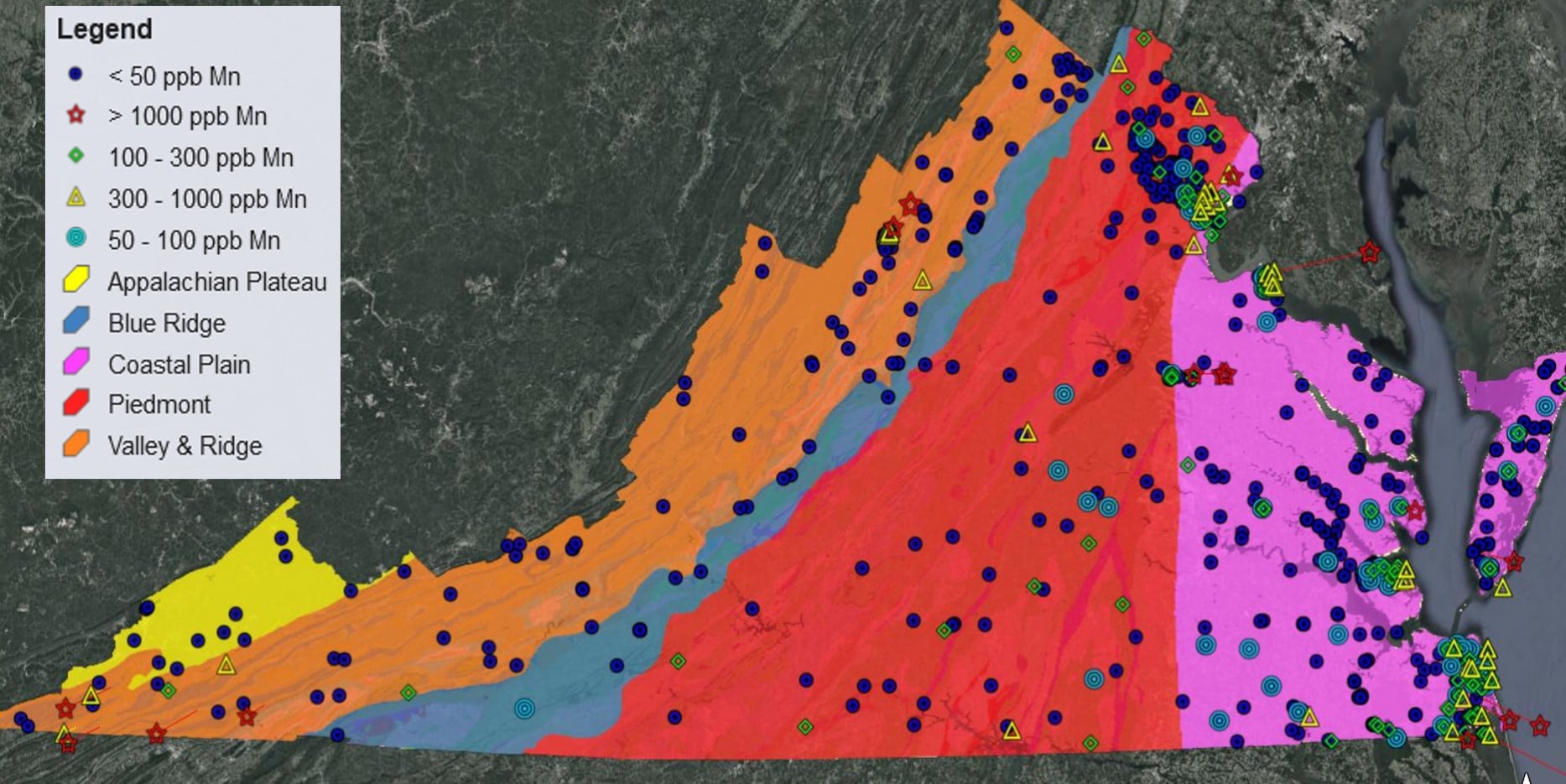
Figure 1: Mn well water data (obtained from McMahon et al., 2018a) plotted for the state of Virginia along with the Physiographic Provinces of Virginia, overlying the Virginia Geologic Map (Sources: McMahon et al., 2018a, Shufeldt et al., 2012).
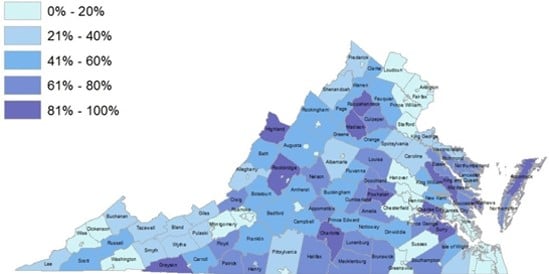
Figure 2: Percent of population in Virginia counties that rely on private wells for drinking water. (Source: Virginia State Water Resources Plan, 2015).
Mn contaminated groundwater wells are found in the upper portion of the Shenandoah Valley and the southernmost region of Southwest Virginia (Figure 1). Potential sources of Mn and heavy metal contamination in Virginia groundwater include: dissolution of the aquifer, soil-water or sediment-water interactions, Mn mobilization via desorption from mineral surfaces in the soil-water interface, heavy metal inputs from past mining, acidic mine waters dissolving heavy metals, etc. Recent research on Mn contamination in the Roanoke river watershed points to mobilization of Mn associated with carbonate-rich rocks (Kiracofe et al. 2017). Two other recent studies investigating Mn groundwater contamination, one in the Piedmont region of North Carolina (Gillispie et al., 2016) and one surveying the entire United States (McMahon et al., 2018b) both found that soil geochemistry is strongly linked to Mn concentrations in groundwater. It is thus also possible that land use patterns may contribute to Mn contamination of groundwater. For example, agricultural tilling or disturbances from ore mining in the Shenandoah Valley could increase soil weathering rates which would in turn enhance mobilization of Mn and other heavy metals from the solid to the aqueous phase. Coal mine drainage, which is primarily localized to Southwest Virginia (Figure 3), can also mobilize heavy metals, including Mn (Figure 4). In order to predict the likelihood of Mn (or other heavy metal) contamination in any drinking water source, identifying the major controls on their concentrations is critical.
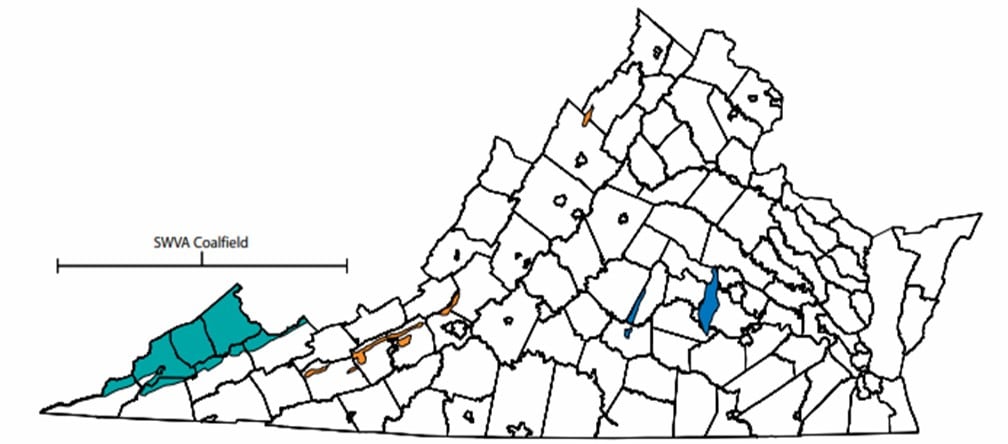
Figure 3: Coalfields of Virginia, with the Southwest Virginia Coalfield in teal, the Valley Coalfields in orange, and the Eastern Coalfields in Blue (Source: Gilmer et al. 2005).

Figure 4: Hinkle’s Environmental Field Methods students investigating acid mine drainage remediation site in West Virginia, with its characteristic orange-red coloration due to Fe oxidation (A) and a coal mine drainage remediation site in Pennsylvania with clear water, yet substantial Mn and heavy metal contamination (B) (Source: Hinkle).
We propose to investigate the role of land use patterns, soil geochemistry, aquifer type, and nearby stream compositions on Mn and other heavy metal concentrations in ground and surface waters in the Shenandoah Valley and Southwest regions of Virginia. These two regions were selected because of the high concentrations of Mn in several wells in these areas (McMahon et al. 2018a), differences in agricultural and land use practices, and potentials for Mn and heavy metal inputs from coal mining and historical ore mining operations. While there is already a substantial amount of well water data accessible via the USGS National Water Information Network (NWIN) and the Virginia Household Water Quality Program (VAHWQP), Mn concentrations in springs are not as well documented, even though springs are used as private drinking water sources. Thus, we propose to collect and analyze spring waters to add to the well water databases, to collect and analyze surface waters and soil cores near groundwater wells and springs, and to use sediment magnetism to characterize land use and soil pollution.
Water compositions will be determined by a combination of ion chromatography (IC) and inductively-coupled plasma-mass spectrometry (ICP-MS) as well as in the field pH, dissolved oxygen, specific conductivity measurements, and alkalinity titrations. Soils will be analyzed by scanning electron microscopy paired with energy dispersive X-ray spectroscopy (SEM-EDS) and x-ray fluorescence to determine particle morphologies and general elemental compositions. Overall soil geochemistry will be analyzed by sequential extractions to determine the fractions that are exchangeable, adsorbed or associated with carbonates, adsorbed to Fe and Mn oxides, associated with organic matter (OM) and sulfide minerals, and the residual fraction. The speciation of Mn in the soils (i.e., does the Mn primarily occur as Mn oxides, bound to clays or Fe oxides, associated with OM, or in solution as aqueous Mn) will be determined by X-ray absorption near edge structure (XANES) spectroscopy at a synchrotron radiation light source. The role of geology and aquifer type will be assessed by plotting the wells and springs in ArcGIS to determine the underlying geology and correlate well depth with the specific aquifer. Because field sites have a variety of land use histories, there is also a unique opportunity to compare magnetic signatures from Fe oxides to land use patterns and Mn contamination. This will further develop the library of magnetic coercivity spectra used to analyze soil and other sediments.
Potential Student Projects
All students will participate in field work, soil and water sample collection during the first two to three weeks of the summer research period. We expect that the students will gravitate toward one facet of the project and that, along with the student and their advisor’s background, will help them choose a specific topic to investigate more deeply. All students will learn scanning electron microscopy and/or X-ray fluorescence to use as needed for their project. Students will use these techniques to better understand soil and sediment morphology and composition. While we envision the possibility of these four projects to address the research questions at hand, some projects can be combined and all projects will share data and interpretations.
Students will be grouped into teams of two, with each student taking on one prong of that research. The teams and breakdowns of projects are:
Team 1: What is the role of past and present land use practices on soil magnetic signatures?
Two students will analyze soil samples collected from sites near groundwater wells and springs, with one student focusing on samples collected from the Shenandoah Valley and one on samples from SW Virginia. These students will accompany Lindquist to the Institute for Rock Magnetism at the University of Minnesota where the samples will be analyzed for magnetic indicators of land use and Mn pollution. This work will focus on using magnetic measurements to determine the morphology and type of iron oxides present in the soil using magnetic demagnetization spectra. Iron oxides are highly sensitive to soil conditions and are a common pollution product, thus the nature of the iron oxides in these soils will help us better understand past and present soil conditions.
Team 2: What is the soil composition near waters that have characteristically high Mn concentrations? Does that differ from soils near waters with low/non-existent Mn?
Two students will perform sequential extractions and complete acid digestions on soil samples collected from sites identified as having Mn concentrations > 100 ppb and Mn concentrations < 50 ppb. These students will also prepare standards and samples for analysis by ICP-OES and analyze the ICP-OES data. The students in this group will also prepare soil samples for XANES measurements and attend Beam Time with Hinkle in Fall 2020 to gain experience in synchrotron-based science. Their work will identify what contaminants are present in the soils (if any), their relative concentrations, and how labile those contaminants are. If, as it is suggested by Gillispie et al. (2016) and McMahon et al. (2018b), weathering from soils is correlated with Mn contamination in groundwater, this work will serve to help identify the extent of that process in Virginia drinking waters. One student will focus on samples from the Shenandoah Valley and one will focus on samples from SW VA.
Team 3: What is the geochemical composition of drinking water (surface, spring, and well water) with elevated Mn? With low to no Mn?
Two students will analyze the data the group obtained from field measurements of the water chemistry (e.g., pH, dissolved oxygen, specific conductivity, and alkalinity titrations) associated with each site visited. The students will also prepare collected water samples and standards for ICP-MS (for 27 elements: Mn, Fe, As, antimony, barium, beryllium, lead, mercury, thallium, nickel, chromium, phosphorous, sulfur, strontium, cadmium, cobalt, zinc, copper, fluoride, aluminum, titanium, zircon, boron, silver, lithium, silicon, and selenium) and IC analysis (for cations like sodium and magnesium and anions like phosphate, sulfate, and nitrate) as well as analyze and interpret the data they obtain. It is quite possible that there will be correlations between the aqueous Mn and other heavy metals, as has been identified for Mn and As in well waters in North Carolina (Sanders et al., 2014). In the Coastal Plain region of Virginia, it has already been documented that there are positive correlations aqueous Mn and hard water (water with high concentrations of calcium, magnesium, and carbonate ions due to the dissolution of carbonate host rocks) in wells (McFarland, 2010), and it is possible that this trend may be consistent across the state. The data from these analyses will be paired with their geographic location and well depth so that water contamination can be spatially resolved across the state. Work will be divided between the students based on geographic location (e.g., Shenandoah Valley and SW VA).
Team 4: Are there correlations between heavy metal concentrations in drinking water and the geologic, pedologic, hydrologic, or land use history of that site?
Two students will be responsible for maintaining the database for water and soil geochemical data, paired with the site’s geographic location. Using ArcGIS or QGIS and well depth information, students will identify the surface geology, physiographic province, soil type, distance to the nearest surface water body, and aquifer for each site, and plot each site with its corresponding attribute table (with water and soil geochemical data). They will generate maps with our data on heavy metal contamination (such as Mn, As, or lead) as well as data from other sources such as the USGS NWIN and the VAHWQP. If the student is particularly motivated and has strong support from an academic advisor interested in logistic regression modeling, they can use the data to determine if any relationships exist between geologic, pedologic, or hydrologic settings and heavy metal concentrations in the groundwater, as has been done for As in Virginia (VanDerwerker et al., 2018). One student will focus on the Shenandoah Valley & one will focus on SW VA.
PROJECT LOGISTICS
- Week 1: Students arrive at Washington & Lee University, readings and introduction to the project and region, selecting project, training in sample collection and begin sample collection at field sites
- Week 2: Sample collection at field sites
- Week 3: Complete sample collection at field sites, return to Washington & Lee University, begin sample prep
- Week 4: SEM/EDS, analyzing field data, preliminary analyses, abstract writing
Field sites include:
Roanoke, Virginia (Cave Spring)
Bath County, Virginia (Bubbling Springs, Warm Springs, Hot Springs, Healing Springs)
Augusta County (Augusta Springs and Wetland)
Lee County (Ely Creek Watershed)
Rockbridge County (Peniel Farm, Big Spring, Magnesia Spring, Old Distillery Spring, Warm Spring, Cold Spring, Holmes Spring, School House Spring, and/or other springs identified by Hinkle as worth investigating as a result of a volunteer project she is currently aiding)
Shenandoah County (Seven Springs)
Summer 2020 will involve both field work and lab work, primarily located at Washington & Lee University in Lexington, VA. Students will stay in on-campus housing at Washington & Lee University with other summer research students. Meals will be available for purchase (covered via Keck funding) from on-campus dining options. Once on campus, we will begin by spending half days delving into the literature surrounding groundwater contamination and the remainder of the day at nearby field sites to learn sample collection techniques and field instrumentation. All students will become proficient in water sample collection, soil sample collection, and aqueous geochemical field methods. During this initial stage, students will select their projects.
Week 2 will be focused on sample collection at all field sites, wrapping sample collection up during Week 3. Transportation from Lexington, VA to field sites will be via van, with most field sites within a 3 hour drive from Lexington, VA. The remainder of Week 3 will be spent on sample and standard preparation for analyses conducted during Week 4. During this time, students will also concentrate on their own research proposals and develop a timeline for tasks to be completed during the academic 2020-2021 year.
We will base our lab work out of the Environmental Geochemistry Lab at Washington & Lee, with classrooms and a computer lab (with ArcGIS & QGIS installed) available for work and group discussions. The Integrative & Quantitative (IQ) Center at Washington & Lee houses the SEM/EDS, which all students will learn to use for soil sample imaging and elemental analysis, with Teams 1 & 3 becoming proficient in its use during Week 4.
After the summer, during the academic 2020-2021 year, students will continue progressing with data collection and analysis as stipulated by their research proposals and timelines. We will touch base in a monthly whole-group videoconference and arrange to check in with individual students and their home advisor (when possible) every month (or as needed) so that goals are met and any issues are addressed.
Safety
This project involves field work and lab work, both of which pose potential safety concerns. Possible risks with field work include, but are not limited to: dehydration, heat stroke, exhaustion, sunburn, insect bites, poisonous snakes or spiders, physical injuries (e.g., sprained ankles, broken bones, lacerations, skin abrasions). Traveling to field sites requires transportation in vehicles, therefore potential personal injury due to vehicular accidents during travel to and from field sites is possible. Laboratory work will primarily involve diluting water samples or grinding soils samples to prepare for analysis, but some students will work with acids to stabilize samples or digest samples. All students who work in the Environmental Geochemistry Laboratory undergo safety training that includes the necessary personal protective equipment (PPE) required for their work (lab coats, goggles, nitrile and rubber gloves are supplied by the Environmental Geochemistry Laboratory), as well as the specific actions to take upon spillage or exposure to any chemicals that they may use. Safety Data Sheets (SDS) for all chemicals in the laboratory are compiled in an easy-to-access binder in the laboratory.
PROFESSIONAL DEVELOPMENT
Students will present their findings at the American Geophysical Union Fall Meeting in 2020 or at the Geological Society of America 2020 Annual Meeting, as appropriate depending on sessions that are available. Regardless of the meeting selected, students will work together in teams of at least two to write abstracts and design posters for these presentations. We will also take this time to discuss results, touch base on progress and the timeline for completing the Advanced projects. We will also discuss career plans and paths forward. Students will participate in student activities at these meetings and attend talks in their areas of interest.
All students will submit a short contribution to the Proceedings of the Keck Geology Consortium 2021 Volume, with drafts due to their home advisor by Mid-February 2021, revised drafts to the project directors by March 1st, 2021, with the final paper submitted in Mid-March 2021.
References
Bjørklund, G., Chartrand, M.S., Aaseth, J., 2017. Manganese exposure and neurotoxic effects in children. Environmental Research 155, 380–384.
Borch, T., Kretzschmar, R., Kappler, A., Cappellen, P.V., Ginder-Vogel, M., Voegelin, A., Campbell, K., 2010. Biogeochemical redox processes and their impact on contaminant dynamics. Environmental Science & Technology 44, 15–23.
Bouchard, M.F., Laforest, F., Vandelac, L., Bellinger, D., Mergler, D., 2007. Hair manganese and hyperactive behaviors: Pilot study of school-age children exposed through tap water. Environmental Health Perspectives 115, 122–127.
Bouchard, M.F., Sauvé, S., Barbeau, B., Legrand, M., Brodeur, M.-È., Bouffard, T., Limoges, E., Bellinger, D.C., Mergler, D., 2011. Intellectual impairment in school-age children exposed to manganese from drinking water. Environmental Health Perspectives 119, 138–143.
Brown, G.E., Henrich, V., Casey, W., Clark, D., Eggleston, C.M., 1999. Metal oxide surfaces and their interactions with aqueous solutions and microbial organisms. Chemical Reviews 99, 77–174.
Brown, G.E., Parks, G.A., 2001. Sorption of trace elements on mineral surfaces: Modern perspectives from spectroscopic studies, and comments on sorption in the marine environment. International Geology Review 43, 963–1073.
Cornell, R.M., Schwertmann, U., 2003. The iron oxides: Structure, properties, reactions, occurrences and uses. WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim., 712 p.
Dixit, S., Hering, J.G., 2003. Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: Implications for arsenic mobility. Environmental Science & Technology 37, 4182–4189.
Fuller, C.C., Harvey, J.W., 2000. Reactive uptake of trace metals in the hyporheic zone of a mining-contaminated stream, Pinal Creek, Arizona. Environmental Science & Technology 34, 1150–1155.
Gillispie, E.C., Austin, R.E., Rivera, N.A., Bolich, R., Duckworth, O.W., Bradley, P., Amoozegar, A., Hesterberg, D., Polizzotto, M.L., 2016. Soil weathering as an engine for manganese contamination of well water. Environmental Science & Technology 50, 9963–9971.
Hafeman, D., Factor-Litvak, P., Cheng, Z., van Geen, A., Ahsan, H., 2007. Association between manganese exposure through drinking water and infant mortality in Bangladesh. Environmental Health Perspectives 115, 1107–1112.
Hlawatsch, S., Garbe-Schönberg, C.D., Lechtenberg, F., Manceau, A., Tamura, N., Kulik, D.A., Kersten, M., 2002. Trace metal fluxes to ferromanganese nodules from the western Baltic Sea as a record for long-term environmental changes. Chemical Geology 182, 697–709.
Islam, F.S., Gault, A.G., Boothman, C., Polya, D.A., Charnock, J.M., Chatterjee, D., Lloyd, J.R., 2004. Role of metal-reducing bacteria in arsenic release from Bengal delta sediments. Nature 430, 68–71.
Jenne, E.A., 1968. Controls on Mn, Fe, Co, Ni, Cu, and Zn concentrations in soils and water: The significant role of hydrous Mn and Fe oxides, in: Advances in Chemistry. American Chemical Society, pp. 337–387.
Khan, K., Wasserman, G.A., Liu, X., Ahmed, E., Parvez, F., Slavkovich, V., Levy, D., Mey, J., van Geen, A., Graziano, J.H., Factor-Litvak, P., 2012. Manganese exposure from drinking water and children’s academic achievement. NeuroToxicology 33, 91–97.
Kiracofe, Z.A., Henika, W.S., Schreiber, M.E., 2017. Assessing the geological sources of manganese in the Roanoke River Watershed, Virginia. Environmental Engineering Science 23, 43–64.
Kumar, E., Bhatnagar, A., Hogland, W., Marques, M., Sillanpää, M., 2014. Interaction of inorganic anions with iron-mineral adsorbents in aqueous media–a review. Advances in Colloid and Interface Science 203, 11–21.
Langley, R., Kao, Y., Mort, S., Bateman, A., Simpson, B., Reich, B., 2015. Adverse neurodevelopmental effects and hearing loss in children associated with manganese in well water, North Carolina, USA. Journal of Environmental and Occupational Science 4, 62.
Lion, L.W., Altmann, R.S., Leckie, J.O., 1982. Trace-metal adsorption characteristics of estuarine particulate matter: evaluation of contributions of iron/manganese oxide and organic surface coatings. Environmental Science & Technology 16, 660–666.
Liu, J., Durand, J.P., Espinal, L., Garces, L.-J.-. J., Gomez, S., Son, Y.-C.-. C., Villegas, J., Suib, S.L., 2004. Layered manganese oxides: synthesis, properties, and applications, in: Handbook of Layered Materials. p. 475.
Ljung, K., Vahter, M., 2007. Time to re-evaluate the guideline value for manganese in drinking water? Environmental Health Perspectives 115, 1533–1538.
Manceau, A., Tamura, N., Celestre, R.S., MacDowell, A.A., Geoffroy, N., Sposito, G., Padmore, H.A., 2002a. Molecular-scale speciation of Zn and Ni in soil ferromanganese nodules from loess soils of the Mississippi Basin. Environmental Science & Technology 37, 75–80.
Manceau, A., Tamura, N., Marcus, M.A., MacDowell, A.A., Celestre, R.S., Sublett, R.E., Sposito, G., Padmore, H.A., 2002b. Deciphering Ni sequestration in soil ferromanganese nodules by combining X-ray fluorescence, absorption, and diffraction at micrometer scales of resolution. American Mineralogist 87, 1494–1499.
McMahon, P.B., Belitz, K., Reddy, J.E., Johnson, T.D., 2018a. Elevated manganese concentrations in United States groundwater, role of land surface–soil–aquifer connections. Environmental Science & Technology 53, 29–38.
McMahon, P.B., Reddy, J.E., Johnson, T.D., 2018b. Data for elevated manganese concentrations in United States groundwater, role of land surface-soil-aquifer Connections. U.S. Geological Survey Data Release.
Nelson, Y.M., Lion, L.W., 2003. Formation of biogenic manganese oxides and their influence on the scavenging of toxic trace elements, in: Geochemical and Hydrological Reactivity of Heavy Metals in Soils. CRC Press. pp. 169–186.
Peacock, C.L., Sherman, D.M., 2007. Sorption of Ni by birnessite: Equilibrium controls on Ni in seawater. Chemical Geology 238, 94–106.
Post, J.E., 1999. Manganese oxide minerals: Crystal structures and economic and environmental significance. Proceedings of the National Academy of Sciences of the United States of America 96, 3447–3454.
Sanders, A.P., Desrosiers, T.A., Warren, J.L., Herring, A.H., Enright, D., Olshan, A.F., Meyer, R.E., Fry, R.C., 2014. Association between arsenic, cadmium, manganese, and lead levels in private wells and birth defects prevalence in North Carolina: A semi-ecologic study. BMC Public Health 14, 955.
Smith, J.D., Longmore, A.R., 1980. Behaviour of phosphate in estuarine water. Nature 287, 532–534.
Spangler, A.H., Spangler, J.G., 2009. Groundwater manganese and infant mortality rate by county in North Carolina: An ecological analysis. EcoHealth 6, 596–600.
Spangler, J.G., Reid, J.C., 2010. Environmental manganese and cancer mortality rates by county in North Carolina: An ecological study. Biological Trace Element Research 133, 128–135.
Tebo, B.M., Ghiorse, W.C., van Waasbergen, L.G., Siering, P.L., Caspi, R., 1997. Bacterially mediated mineral formation; insights into manganese (II) oxidation from molecular genetic and biochemical studies. Reviews in Mineralogy and Geochemistry 35, 225–266.
U.S. Environmental Protection Agency, 2004. Drinking Water Health Advisory for Manganese. Washington, D. C. EPA-822-R-04-003
VanDerwerker, T., Zhang, L., Ling, E., Benham, B., Schreiber, M., 2018. Evaluating geologic sources of arsenic in well water in Virginia (USA). International Journal of Environmental Research and Public Health 15, 787.
Williams, M., Todd, G.D., Roney, N., Crawford, J., Coles, C., McClure, P.R., Garey, J.D., Zaccaria, K., Citra, M., 2012. Toxicological Profile for Manganese. U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry. 506 p.
Young, L.B., Harvey, H.H., 1992. The relative importance of manganese and iron oxides and organic matter in the sorption of trace metals by surficial lake sediments. Geochimica et Cosmochimica Acta 56, 1175–1186.

